DAMAIRA Pharmaceuticals Pvt. Ltd. is a fast-emerging pharmaceutical company that is setting up a world-class EU and USFDA-compliant Dry Powder Injectable manufacturing facility. This upcoming facility focuses on Carbapenem Dry Powder Injectables, aiming to revolutionize the pharmaceutical industry with state-of-the-art technology, rigorous quality control processes, and highly advanced manufacturing systems. The facility is poised to become a global leader in sterile pharmaceutical production, emphasizing high standards of safety, precision, and quality.
DAMAIRA Pharmaceuticals is now looking for talented professionals to join their growing team. Walk-in interviews are being held for multiple positions across various departments on 22nd September 2024.
Company Overview – DAMAIRA Pharmaceuticals Pvt. Ltd.
DAMAIRA Pharmaceuticals is dedicated to becoming a global player in the field of Carbapenem Dry Powder Injectables, an important segment in the treatment of bacterial infections. Their facility is equipped with the latest technology and systems like SCADA 21 CFR, ensuring compliance with global standards, such as EU and USFDA regulations. The company has invested in premium machinery, ensuring high-quality production with multiple checks and safeguards in place to minimize the chances of error.
With systems like Water Generation Plants and advanced sterile processing environments, DAMAIRA is committed to delivering safe, effective, and reliable pharmaceuticals. The company’s goal is to achieve accreditation from the top regulatory agencies globally, and it invites dynamic professionals to join them in this exciting journey.
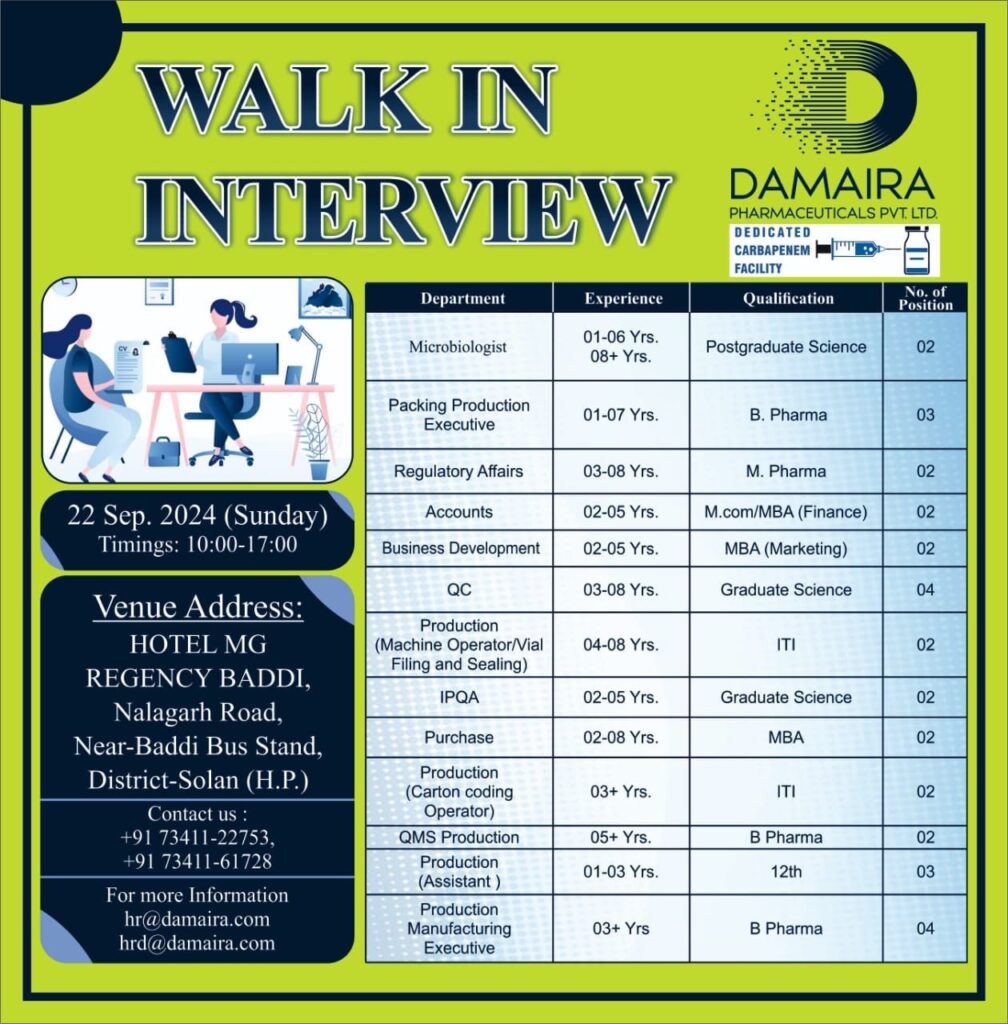
Walk-In Interview – Details
- Date: 22nd September 2024 (Sunday)
- Timing: 10:00 AM to 4:00 PM
- Venue: Hotel MG Regency Baddi, Nalagarh Road, Near Baddi Bus Stand, District-Solan, Himachal Pradesh.
Positions Available:
DAMAIRA Pharmaceuticals is hiring for various roles across different departments. The available departments include:
- Production
- Packing
- Microbiology
- Quality Control (QC)
- IPQA (In-Process Quality Assurance)
- Regulatory Affairs
- Quality Management Systems (QMS)
- Accounts
- Purchase
- Business Development
Eligibility Criteria:
- Minimum Qualifications:
- B.Pharm, M.Pharm
- I.T.I, M.Com, Graduate/ Post Graduate (Science)
- MBA, 12th Pass
- Experience:
- Candidates with 1 to 8+ years of experience in their respective fields are eligible to apply.
- Job Location:
- Panchkula, Haryana
Key Roles and Responsibilities by Department:
- Production:
- Overseeing the manufacturing of Dry Powder Injectables.
- Ensuring adherence to Good Manufacturing Practices (GMP) and quality control standards.
- Managing the production line and ensuring efficiency.
- Packing:
- Handling the packaging of pharmaceuticals, ensuring that all products are packed according to specifications and compliance.
- Coordinating with production to maintain the supply chain flow.
- Microbiology:
- Ensuring sterile conditions are maintained throughout the production process.
- Conducting microbial testing and ensuring that the facility adheres to microbiological standards.
- Quality Control (QC):
- Performing quality checks on raw materials and finished products.
- Monitoring product quality through rigorous testing and analysis.
- In-Process Quality Assurance (IPQA):
- Ensuring that production processes meet regulatory and internal quality standards at every stage.
- Real-time monitoring of the manufacturing process to prevent errors.
- Regulatory Affairs:
- Handling the preparation and submission of regulatory documents to comply with EU, USFDA, and other international standards.
- Managing communication with regulatory authorities and staying updated on global regulatory changes.
- Quality Management Systems (QMS):
- Developing and maintaining the company’s quality management policies and procedures.
- Conducting internal audits and preparing the facility for external audits.
- Accounts:
- Managing the financial activities of the company, including budgeting, reporting, and compliance with financial regulations.
- Overseeing audits and managing payroll.
- Purchase:
- Managing procurement processes, including sourcing raw materials and negotiating with suppliers.
- Ensuring timely availability of materials for production.
- Business Development:
- Identifying new business opportunities and markets.
- Developing partnerships and strategies to expand the company’s reach globally.
Why Join DAMAIRA Pharmaceuticals?
- State-of-the-Art Facility:
- DAMAIRA Pharmaceuticals is leveraging cutting-edge technology to ensure that their facility is at the forefront of pharmaceutical manufacturing. This includes systems like SCADA 21 CFR, making the production and quality control processes foolproof.
- Global Compliance:
- DAMAIRA Pharmaceuticals is set to become EU and USFDA compliant, opening doors to international markets. This presents an opportunity to work with a company that adheres to the highest global standards.
- Career Growth:
- Working with DAMAIRA Pharmaceuticals provides a platform for professional development. The company offers opportunities for growth and learning, allowing you to build a successful career in the pharmaceutical industry.
- Contribute to a Revolution in Healthcare:
- DAMAIRA Pharmaceuticals is on a mission to revolutionize the production of Carbapenem Dry Powder Injectables, a critical component in fighting bacterial infections. By joining the team, you will be part of a company that is making a global impact on healthcare.
Required Documents for Walk-In Interview:
Candidates attending the walk-in interview are requested to bring the following documents:
- Updated Resume
- Photocopies of Educational Certificates
- Experience Certificates
- Latest Salary Slips
- Aadhar Card or Any Other ID Proof
- Passport Size Photographs
Contact Details:
- Phone: +91 73411-22753
- Email: hr@damaira.com
Conclusion:
DAMAIRA Pharmaceuticals Pvt. Ltd. offers a unique opportunity to be part of a pioneering pharmaceutical venture that is set to make a mark on the global stage. With multiple positions available across various departments, the company is seeking skilled professionals who are passionate about contributing to the future of healthcare. The walk-in interview on 22nd September 2024 is an excellent opportunity to join a forward-thinking organization that values innovation, quality, and excellence.